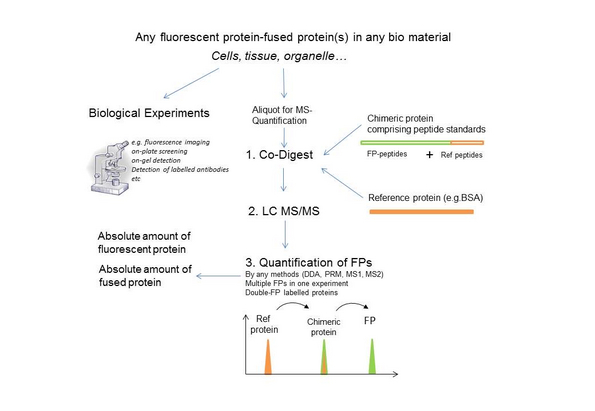
Fusions with fluorescence proteins (FPs) play a pivotal role in experimental biology because of their sensitive and spatially precise visualization by spectroscopy. However, observed fluorescence is not always proportional to their molar abundance. Only a fraction of the fusion protein containing the mature fluorescence chromophore is detectable by spectroscopy and there is no generic method for estimating its molar abundance. We developed a fluorescence-independent mass-spectrometry based approach for accurate absolute sub-femtomole quantification of FP-fusions that also estimates the protein faction having fully matured chromophore.
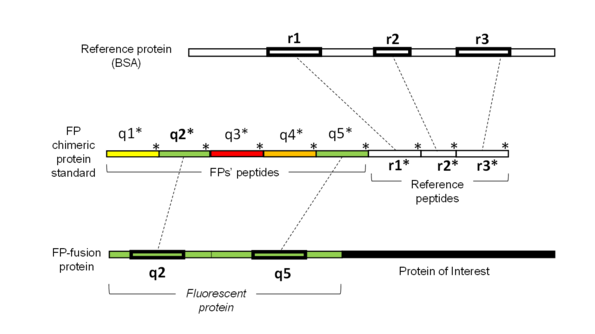
The method relies upon a single synthetic protein standard we called qFP-8 comprising quantotypic peptides from most frequently used FPs e.g. mEGFP, mCherry, mScarlet, Dendra2, mKate2, mNeonGreen, as well as Halo and SNAP small self-labelling protein tags. It enables absolute quantification of more than 70 members from these six FP-families, any of their fusions and proteins labelled with organic chromophores via linkers. Several FPs can be quantified in parallel to determine their molar concentration, expression level and stoichiometric ratio to other (not necessarily FP-fused) proteins. The molar fraction of FPs having the mature chromophore structure is determined in the same analysis.
Archishman Ghosh, Dora Y. Tang (MPI CBG, current affiliation University of Saarland)
Aliona Bogdanova, Eric Geertsma (PEPC Facility, MPI CBG)
DOI: 10.1007/978-1-0716-4462-1_6
Patrick M McCall#, Kyoohyun Kim, Anna Shevchenko, Martine Ruer-Gruß, Jan Peychl, Jochen Guck, Andrej Shevchenko, Anthony Hyman, Jan Brugués#
A label-free method for measuring the composition of multicomponent biomolecular condensates.
Nat Chem, Art. No. doi: 10.1038/s41557-025-01928-3 (2025)
Anna Shevchenko, Archishman Ghosh, Andrea Schuhmann, Aliona Bogdanova, Henrik Thomas, Viditha Rao, Eric R. Geertsma, T-Y. Dora Tang, Andrej Shevchenko Absolute Quantification of Fluorescent Protein Fusions by Proteomics. Pre-print BioRxiv Doi: 10.1101/2025.09.19.677389v1
Contact: ashevche@mpi-cbg.de