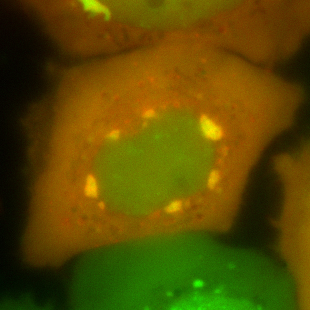
Stress granules (in green) co-localizing with a misfolded protein, the ALS-linked variant of SOD1 (in red).
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that became commonly known through the „Ice Bucket Challenge“. Researchers of the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden found that certain misfolded proteins linked to this disease aggregate specifically in stress granules, membrane-less compartments of the cell. This triggers a decrease of the dynamics of these compartments, changes their composition, and leads to an aberrant liquid-to-solid transition – the granules turn into pathological aggregates. "The results of our study will help us understand how the disease can develop and how different factors can contribute to it," says Daniel Mateju, first author and PhD student in the research group of Simon Alberti at the MPI-CBG.
The research team could also show that cells use a kind of quality control system: Chaperone proteins constantly monitor the composition of stress granules and prevent a conversion into an aberrant state. In case stress granules lose their dynamic state and turn into aggregates nonetheless, chaperone proteins are recruited to these aberrant granules and make sure they are disassembled. This finding may also have relevance for ALS and related age-dependent diseases, because the activity of chaperones is presumed to decline with increasing age. Thus, boosting the chaperone machinery in aging cells may be a new avenue for therapeutic intervention.
Daniel Mateju ,Titus M Franzmann, Avinash Patel, Andrii Kopach, Edgar E Boczek, Shovamayee Maharana, Hyun O Lee, Serena Carra, Anthony A Hyman and Simon Alberti:
An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function
The EMBO Journal, 4 April 2017