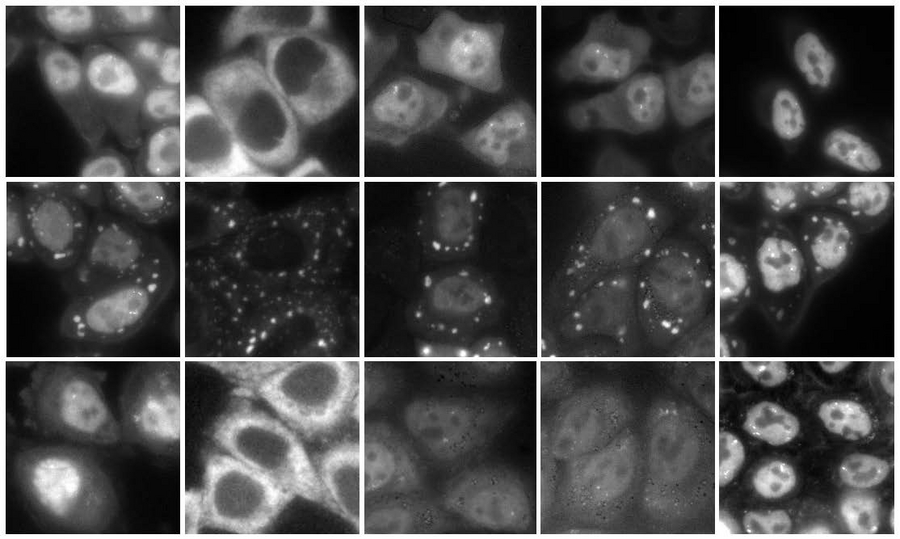
Some proteins, usually diffused in cells (upper panels), form into cytoplasmic punctate condensates upon cellular stresses, called stress granules (middle). Lipoamide specifically prevents condensation of stress granule proteins, which puts them back to their original places (bottom). © Uechi et al., Nat Chem Biol (2025) / MPI-CBG
A collaborative team from the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden and the University of Edinburgh has identified a small molecule that may open a new therapeutic path for neurodegenerative diseases like amyotrophic lateral sclerosis (ALS). The study, led by Anthony Hyman (MPI-CBG) and Richard Wheeler (University of Edinburgh) and published in Nature Chemical Biology, reports that the compound lipoamide can prevent the formation of pathological stress granules—protein-rich condensates associated with cellular stress and ALS.
ALS is a devastating neurodegenerative disorder that progressively destroys motor neurons, leading to paralysis and, ultimately, death. Despite intense research efforts, treatment options remain limited and largely ineffective at halting disease progression. One emerging area of investigation involves stress granules—membraneless condensates that form in response to stress and typically dissolve once the cell recovers. However, in ALS, some components of these granules, including proteins such as FUS and TDP-43, become abnormally trapped in the cytoplasm and form persistent aggregates. These aggregates are thought to disrupt gene regulation and DNA repair by sequestering key proteins away from the nucleus.
“We were looking for non-toxic compounds that could dissolve stress granules without affecting other cellular functions,” explains Hiroyuki Uechi, the first author of the study. “We found that lipoamide modulates the redox state of specific stress granule proteins. This keeps them from condensing into granules, allowing them to return to the nucleus and resume their normal roles.”
The team demonstrated that lipoamide treatment improved motor defects in both fly models of ALS and human lab-grown motor neurons carrying ALS-related mutations. The findings suggest that modulating protein condensation could be a promising strategy for therapeutic intervention—not only in ALS, but potentially in other neurodegenerative diseases linked to protein aggregation.
The research was conducted in fly models and human-derived neurons grown in the lab. While these findings are promising, they are not yet ready for clinical application, and further studies will be needed to determine whether similar effects could be observed in humans.
This study also reinforces the broader role of phase separation in health and disease, highlighting how tiny molecules can have outsized effects on cellular organization.
Uechi, Hiroyuki, Sindhuja Sridharan, Jik Nijssen, Jessica Bilstein, Juan M. Iglesias-Artola, Satoshi Kishigami, Virginia Casablancas-Antras, Ina Poser, Eduardo J. Martinez, Edgar Boczek, Michael Wagner, Nadine Tomschke, António M. de Jesus Domingues, Arun Pal, Thom Doeleman, Sukhleen Kour, Eric Nathaniel Anderson, Frank Stein, Hyun O. Lee, Xiaojie Zhang, Anatol W. Fritsch, Marcus Jahnel, Julius Fürsch, Anastasia C. Murthy, Simon Alberti, Marc Bickle, Nicolas L. Fawzi, André Nadler, Della C. David, Udai B. Pandey, Andreas Hermann, Florian Stengel, Benjamin G. Davis, Andrew J. Baldwin, Mikhail M. Savitski, Anthony A. Hyman, and Richard J. Wheeler. Small-molecule dissolution of stress granules by redox modulation benefits ALS models. Nat Chem Biol (2025). https://doi.org/10.1038/s41589-025-01893-5