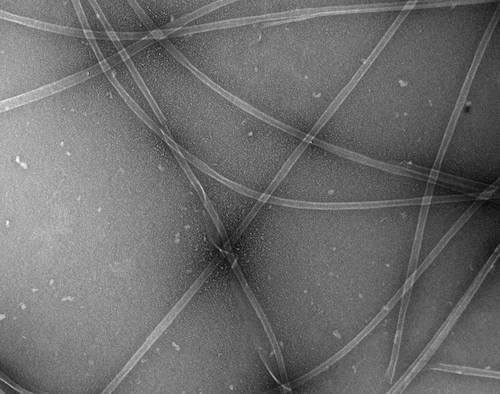
Electron micrograph of microtubules purified from Spodoptera frugiperda cells.
Microtubules are critical cellular structures. They are part of the cytoskeleton that gives the cells its shape. They are important for cell division and chromosome segregation and also make up the network used for transport within the cell. Tubulin is the major building block of microtubules. Both tubulin and microtubules have been intensively studied during the past 50 years. However, during this time, researchers have been faced with a major limitation: tubulin cannot be expressed by standard recombinant methods in the way many proteins, like insulin for example, are produced today. Instead, tubulin is typically purified from animal brain, such as from pigs or cows. This involves going to a local slaughterhouse at dawn to collect fresh brains, which are usually discarded as waste. The brains are then taken back to the lab where they are blended to a kind of ‘shake’ before the tubulin can, step by step, be purified from it. Around two grams of tubulin can be isolated from about 100 pig brains.
Researchers at the MPI-CBG have now developed a simple method to isolate tubulin from potentially any source which makes it possible to study tubulin and associated proteins from any tissue or cell. It exploits the properties of TOG domains, which are domains found in certain class of proteins in cells. The human version, ch-TOG, was originally found to be abundant in specific cancers (hence the name colonic and hepatic tumor-overexpressed gene). Using these TOG domains, tubulin can be selectively bound from any cytoplasm almost like to a magnet. Finally, tubulin is washed out, and, most important, it is active and can form microtubules as the authors show by eletron microscopy.
The new method makes new avenues of research possible: Tubulin and its associated proteins from potentially all model organisms used in research can be specifically studied. The research team initially used cultured cells from the fall armyworm Spodoptera frugiperda as proof of principle. The new method also worked with cells of the frog 0, yeast, C. elegans, and even from human kidney cells. “The latter is particularly important, especially considering that tubulin and microtubules are already targets of a number of cancer drugs”, says co-first author Per Widlund.
The results were published in Molecular Biology of the Cell and were featured on the cover of the journal.
Per O. Widlund, Marija Podolski, Simone Reber, Joshua Alper, Marko Storch, Anthony A. Hyman, Jonathon Howard, and David N. Drechsel:
One-step purification of assembly-competent tubulin from diverse eukaryotic sources
Molecular Biology of the Cell, vol. 23, November 15, 2012