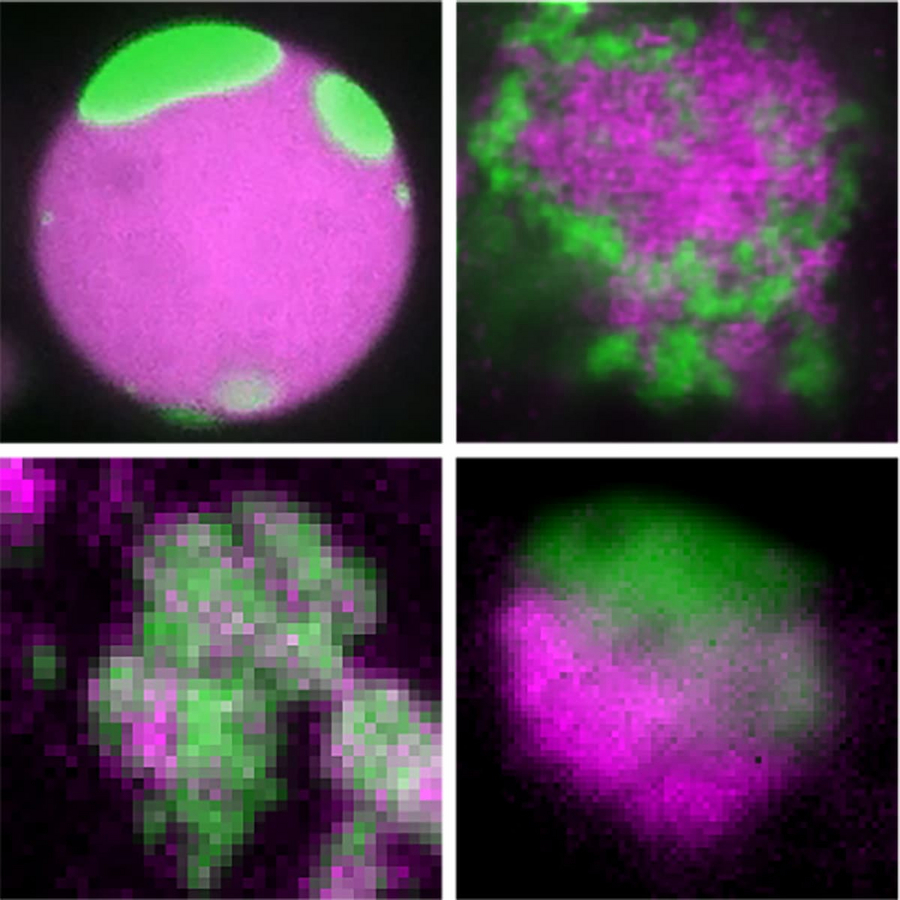
TDP-43 undergoes intra-condensate demixing inside stress granules, leading to pathological aggregation. Shown: TDP-43 demixing (green) in reconstituted granules (magenta) (top left), HeLa cells (top right), iPSC-derived motor neurons (bottom left), and patient brain tissue (bottom right). © Xiao Yan et al. / MPI-CBG
Cells organize many of their internal processes using biomolecular condensates—dynamic, liquid-like droplets that form without membranes. However, condensates are not permanently stable—they transition from a liquid-like to a more solid-like state in a process known as aging. This process is associated with protein aggregation, a hallmark of several neurodegenerative diseases.
But how do aggregates form in the first place—especially when the cellular environment contains a mix of many different proteins? Remarkably, patient samples often reveal aggregates composed almost entirely of a single protein. This raises a fundamental question: How does one protein "recruit itself" from a complex mixture to form a homogeneous aggregate?
To answer this, researchers Xiao Yan, David Kuster, and Jik Nijssen from the lab of Anthony Hyman at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden, together with collaborators from TUD Dresden University of Technology, Texas A&M University, and the Mayo Clinic in Florida, investigated the behavior of TDP-43, a protein whose aggregation is a defining feature of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD).
Under oxidative stress, cells assemble stress granules—mixed condensates containing various proteins and RNAs, including TDP-43. Over time, the researchers observed a striking transformation: TDP-43 separates from the mix, forming distinct condensates within stress granules. They call this process “intra-condensate demixing.” This demixing was seen not only in vitro and in cell models but also in mouse brain tissue and samples from ALS/FTD patients.
Two factors drive this selective demixing. First, TDP-43 concentration must rise above a threshold. Second, oxidative stress alters TDP-43’s structure, enhancing its self-association and reducing its solubility. Once TDP-43 forms its own condensates, these can solidify over time, creating pathological aggregates seen in neurodegeneration.
“Our work shows that stress granules play a crucial role in disease emergence,” says Xiao Yan. “Targeting intra-condensate demixing of TDP-43 may become a promising strategy for therapeutic intervention.”
Xiao Yan, David Kuster, Priyesh Mohanty, Jik Nijssen, Karina Pombo-García, Jorge Garcia Morato, Azamat Rizuan, Titus M. Franzmann, Aleksandra Sergeeva, Anh M. Ly, Feilin Liu, Patricia M. Passos, Leah George, Szu-Huan Wang, Jayakrishna Shenoy, Helen L. Danielson, Busra Ozguney, Alf Honigmann, Yuna M. Ayala, Nicolas L. Fawzi, Dennis W. Dickson, Wilfried Rossoll, Jeetain Mittal, Simon Alberti, Anthony A. Hyman. Intra-condensate demixing of TDP-43 inside stress granules generates pathological aggregates. Cell, May 23, 2025. https://doi.org/10.1016/j.cell.2025.04.039