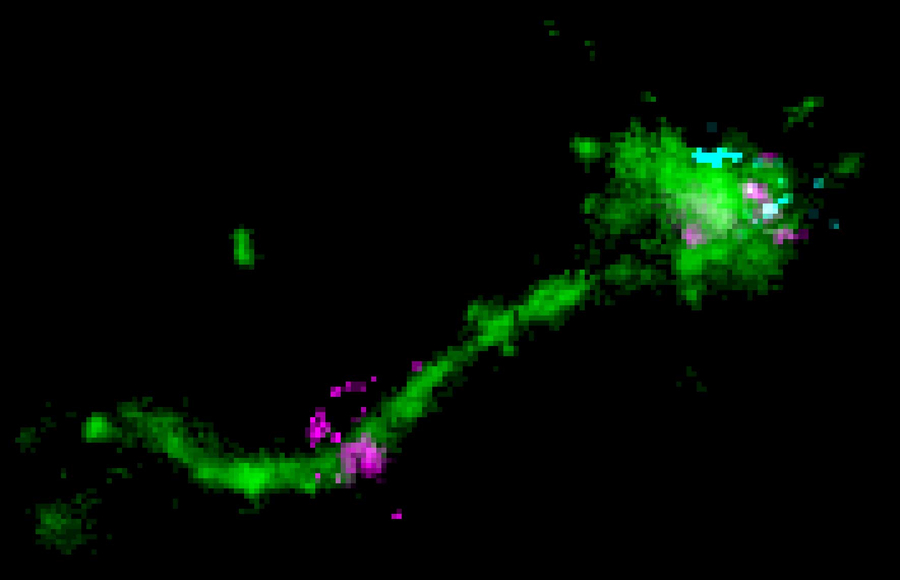
An LNP is located on a long endosomal tubule (green), together with a perpendicular disperse mRNA signal (cyan), and likely representing an instance of mRNA (purple) escape. Copyright: Marino Zerial / MPI-CBG
In recent years, ribonucleic acid (RNA) has emerged as a powerful tool for the development of novel therapies. RNA is used to copy genetic information contained in our hereditary material, the deoxyribonucleic acid (DNA), and then serves as a template for building proteins, the building blocks of life. Delivery of RNA into cells remains a major challenge for the development of novel therapies across a broad range of diseases. Researchers at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden together with researchers from the global biopharmaceutical company AstraZeneca have investigated where and how mRNA is delivered inside the cell. They found that mRNA uses an unexpected entry door. Their results provide novel insights into the development of RNA therapeutics towards efficient delivery and lower dosages.
DNA (deoxyribonucleic acid) contains the genetic information required for the development and maintenance of life. This information is communicated by messenger ribonucleic acid (mRNA) to make proteins. mRNA-based therapeutics have the potential to address unmet needs for a wide variety of diseases, including cancer and cardiovascular disease. mRNA can be delivered to cells to trigger the production, degradation or modification of a target protein, something impossible with other approaches. A key challenge with this modality is being able to deliver the mRNA inside the cell so that it can be translated to make a protein. mRNA can be packed into lipid nanoparticles (LNPs) – small bubbles of fat – that protect the mRNA and shuttle it into cells. However, this process is not simple, because the mRNA has to pass the membrane before it can reach its site of action in the cell interior, the cytoplasm.
Researchers in the team of MPI-CBG director Marino Zerial are experts in visualizing the cellular entry routes of molecules in the cell, such as mRNA with high-resolution microscopes. They teamed up with scientists from AstraZeneca who provided the researchers with lipid nanoparticle prototypes that they had developed for therapeutic approaches to follow the mRNA inside the cell. The study is published in the Journal of Cell Biology.
“To be delivered, the mRNA must make a long journey. Enclosed in the fatty LNP bubble, it needs to get into the cell first,” explains Marino Zerial. “The LNPs arrive at the cell surface where they bind to receptors. They are then taken up into specialized membrane-enclosed compartments called endosomes. At this point, the mRNA is inside the cells but surrounded by two barriers, the fatty bubble and the endosome wall or more correctly, membrane. The challenge for the mRNA is to escape both barriers to reach the cytoplasm where it serves as a template to make proteins. We know that only a tiny fraction of RNA molecules are able to escape into the cytoplasm.” Internalized cargo molecules, like the LNPs, are first transported to "early" endosomes. These are logistic centres that distribute cargo molecules to various destinations in the cell. They either recycle molecules to the cell surface or degrade them in late endosomes and lysosomes. So far, people thought that the mRNA escapes from late endosomes exploiting their very acidic content. “With single molecule microscopy techniques", explains Prasath Paramasivam, the first author of the study, "we could visualize for the first time the mRNA in the LNP inside the endosomes of cells. We also captured the actual escape of the mRNA, which happened in the tubules of the recycling endosomes, which are only mildly acidic." "Our results imply that sending the LNP-mRNA to late endosomes is counterproductive for delivery and only increases cell toxicity." says Zerial. These findings help understanding the mechanism of mRNA escape from endosomes in more detail.
Marino Zerial summarizes: “The LNP delivery system for mRNA necessitates high doses due to the low endosomal escape efficiency. Knowing where the mRNA goes and how it can escape the endosomes allows us to develop better vehicles for more efficient delivery, at lower dosage. We can improve the mRNA delivery system so it can be used for therapeutic applications, for example cancer treatment.”
Prasath Paramasivam, Christian Franke, Martin Stöter, Andreas Höijer, Stefano Bartesaghi, Alan Sabirsh, Lennart Lindfors, Marianna Yanez Arteta, Anders Dahlen, Annette Bak, Shalini Andersson, Yannis Kalaidzidis, Marc Bickle, and Marino Zerial: “Endosomal escape of delivered mRNA from endosomal recycling tubules visualized at the nanoscale”, J Cell Biol (2021): e202103003, doi: 10.1083/jcb.202110137
Katrin Boes
+49 (0) 351 210 2080
kboes(at)mpi-cbg.de
Prof. Marino Zerial
+49 (0) 351 210 1100
zerial(at)mpi-cbg.de