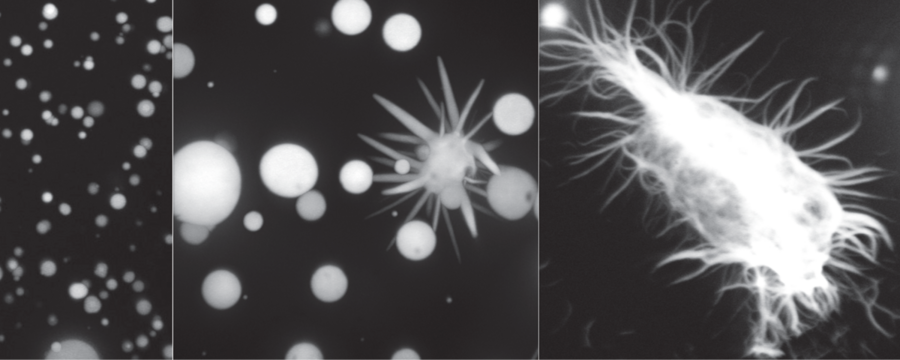
FUS protein liquid droplets formed in a test tube solidifies into aggregates over time - resembling what we see in ALS patient neurons.
Researchers at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) discovered that liquid-like compartments in a cell can become solid over time. Studying the protein FUS – which is associated with the neurodegenerative disease Amyotrophic Lateral Sclerosis (ALS) – they showed that FUS forms liquid compartments but, when aged, converts to a solid state and forms aggregates. This phenomenon is accelerated with mutations of the protein FUS derived from ALS patients, so this aberrant phase transition seems to lie at the heart of ALS and also might be a general mechanism for all neurodegenerative diseases. (Cell, 27 August 2015)
Many functional compartments of a cell are not bound by membranes: They are thought to form by liquid-liquid demixing – like oil in vinegar. The Hyman Lab at the MPI-CBG is interested in understanding proteins that form such liquid compartments. They teamed up with the group of Simon Alberti, who studies proteins that do exactly the opposite: they tend to aggregate, because they contain large disordered regions, known as prion-like proteins. FUS is such a protein and known to be implicated in the neurodegenerative disease Amyotrophic Lateral Sclerosis (ALS).
To look at the behavior of FUS, researchers used a new microscope built for them by Gene Myers and his lab. What they could see: In healthy cells, FUS compartments behave like a liquid. “It’s spherical, molecules can move around inside the droplet, and it fuses with other FUS droplets,” explains Hyun-Ok Lee, postdoc in the lab of Tony Hyman, and one of the first authors. However, FUS forms aggregates in neurons of ALS patients: “When aged in a test tube, the droplets converted to solid aggregates”, says co-author Avinash Patel, also postdoc in the same lab. “That discovery was really striking”. Researchers could show that these aggregates form even faster with the mutations in the FUS protein that are found in ALS patients.
The link to age-related diseases
The story of trying to understand the behavior of FUS is another example of how studying the dynamics of intracellular compartments can lead to insights about neurodegenerative diseases. The fact that liquid compartments can form aggregates over time could explain why many protein aggregates are a hallmark of age-related diseases like ALS, Alzheimer’s, or Parkinson’s. Fighting age-related diseases means to strive for a better quality of life: “I don’t necessarily want people to live longer, but definitely to live happier”, Patel says.
Watch a video explaining the work at www.youtube.com/cellvideoabstracts or directly at youtu.be/8kbOK5N_TvM
Avinash Patel, Hyun O. Lee, Louise Jawerth, Shovamayee Maharana, Marcus Jahnel, Marco Y. Hein, Stoyno Stoynov, Julia Mahamid, Shambaditya Saha, Titus M. Franzmann, Andrej Pozniakovski, Ina Poser, Nicola Maghelli, Loic A. Royer, Martin Weigert, Eugene W. Myers, Stephan Grill, David Drechsel, Anthony A. Hyman, and Simon Alberti:
A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation
Cell, 27 August 2015