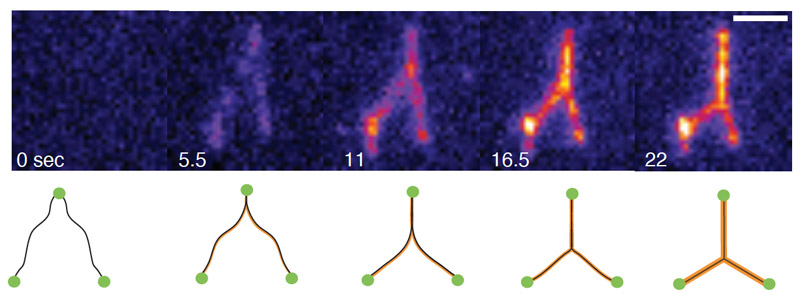
Representative images of FoxA1 zipping two independent DNA strands over time. Scale bar = 2 μm. (Fig.1(g) from the article)
Understanding how individual proteins work together to perform complex cellular processes such as transcription, DNA replication, and repair represents a crucial goal in cell biology. Transcription is a process in the nucleus where protein complexes work together to generate transcripts of RNA from genes. For proper transcriptional regulation, enhancers—short strips of DNA—must be brought into close proximity of the gene’s promoter. Given that enhancers and promoters are often located far apart within the genome, the question then arises: how do proteins bring these enhancers and promoters together in space and time? And what are the physics behind it?
Answering these questions would provide deep insights into the proper regulation of transcription in the cell nucleus. However, extracting such information is far from trivial. But recent work from the research group of Jan Brugués at the Max Planck Institute of Molecular Cell Biology and Genetics in collaboration with Frank Jülicher at the MPI for the Physics of Complex Systems has revealed an important clue: Forces. Jan’s lab is also located at the MPI for the Physics of Complex Systems and is affiliated with the Center for Systems Biology Dresden.
Interactions between liquids and solids have long been known to generate forces, such as those maintaining the tension of a spider web or those that allow insects to walk on water. However, whether such forces play a role inside the cell has remained unclear. With the development of precise biophysical methods and advanced imaging techniques, we are getting closer to not only observing such forces but also measuring them.
The Brugués lab imaged the interactions between single molecules of DNA and the transcription factor FoxA1, a protein responsible for determining cell fate in many species. They discovered that FoxA1 molecules brought distant regions of DNA together, generating forces that condensed the DNA. When the single molecule of DNA was stretched tightly — like a tightened elastic band — FoxA1 molecules could not bring DNA together. However, when the DNA molecule was floppy, FoxA1 molecules worked together to condense the DNA, overcoming the DNA’s intrinsic tension. This new information helps paint a clearer picture of the interactions between transcriptional regulators and the surface of the DNA.
Remarkably, the physics underlying these FoxA1-DNA interactions are reminiscent of the forces that maintain the tension of a spider web. Similar to how liquid droplets on a spider web generate forces that reel in broken strands of silk, FoxA1 acts as the liquid phase that condenses DNA and brings it together.
A gateway to understanding forces
This study demonstrated how proteins work together to generate forces in the cell nucleus. Such a result opens an exciting research direction to understanding other complex processes in the cell. Thomas Quail, post-doctoral researcher in the Brugués lab says: “Our findings set forth a novel mechanism that the cell nucleus may use to organize its chromatin and DNA. It’s possible that these condensation forces generated between solid and liquid surfaces could also be relevant for other cellular bodies such as the mitotic spindle and membranes.”
Thomas Quail, Stefan Golfier, Maria Elsner, Keisuke Ishihara, Vasanthanarayan Murugesan, Roman Renger, Frank Jülicher & Jan Brugués: Force generation by protein–DNA co-condensation. Nat. Phys. (2021), www.doi.org/10.1038/s41567-021-01285-1