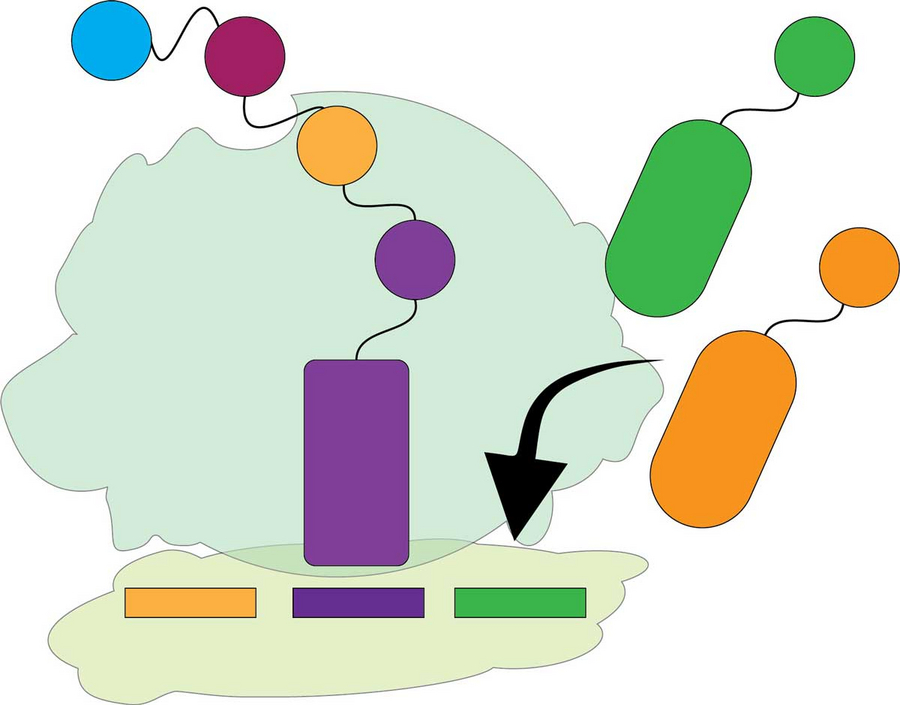
The ribosome translates the mRNA into a protein, which consists of many amino acids. tRNAs carrying these amino acids arrive randomly at the ribosome. The ribosome has to bind the correct tRNA (green) and distinguish it from the wrong one (orange). If the ribosome makes a mistake here, the resulting protein will contain the wrong amino acid. © Landerer et al. Molecular Biology and Evolution, 2024 / MPI-CBG
Cells of organisms usually process genetic information accurately to keep them healthy and functional. But sometimes mistakes can happen during the process of translating genetic information into proteins, one of the the building blocks of our cells. In this case, the wrong amino acid is built into a protein. While most of these mistakes might not affect the health of a cell, some could actually change how a protein works. To understand the effects of these specific mistakes better, it is crucial to know how often these mistakes happen in all the proteins a cell makes and how they affect evolution.
Three researchers, Cedric Landerer, Jonas Poehls and, Agnes Toth-Petroczy from the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) and the Center for Systems Biology Dresden (CSBD), now present the first study that shows how protein-making mistakes are distributed in the proteome (the entire set of proteins), how often they happen, and what the wrong protein means for the health and functionality of a cell.
The first author of the study, Cedric Landerer, explains, “We studied two organisms, the bacterium E. coli and S. cerevisiae (also known as brewer’s or baker’s yeast), and found that about 20–23% of all proteins in these cells could have at least one mistake in them. Interestingly, harmful mistakes were less common. We trained our model using data from mass spectrometry experiments that detected these mistakes.”
“In general, it seems that the cells we studied have a pretty good system in place to handle mistakes during protein-making,” says research group leader Agnes Toth-Petroczy. “With our pipeline and the mechanistic model, we can now explore how accurate protein-making is for many different species. We can also look at how protein-making accuracy changes in different situations, like when cells are stressed or during diseases.”
Cedric Landerer, Jonas Poehls, Agnes Toth-Petroczy: Fitness Effects of Phenotypic Mutations at Proteome-Scale Reveal Optimality of Translation Machinery, Molecular Biology and Evolution, Volume 41, Issue 3, March 2024, https://doi.org/10.1093/molbev/msae048