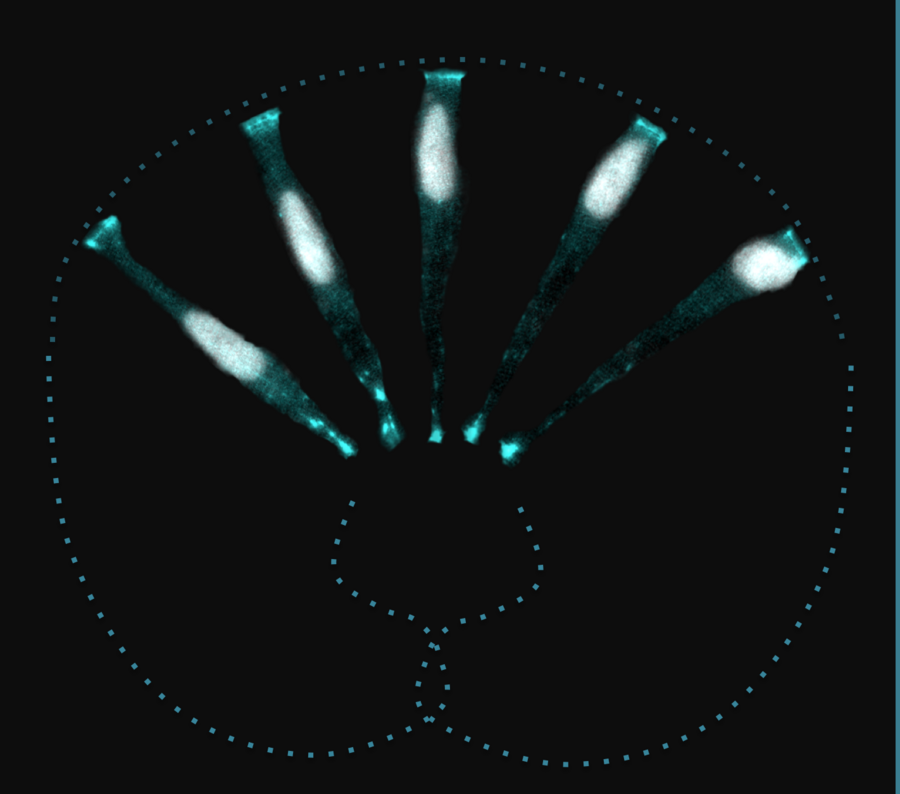
Artistic montage of different cells showing how nuclei move towards the apical side of the zebrafish retinal neuroepithelium. Nuclei are in grey, cell outline in cyan. Copyright: Iskra Yanakieva / MPI-CBG
The correct position of cell nuclei is crucial for cellular function and tissue development. In pseudostratified epithelia (PSE) – a tissue, densely packed with elongated cells and found as precursor of many organs including gut, liver, lung and the brain – it is known that the nuclei have to move towards the apical side of the epithelium before cell division. Otherwise, the tissue does not develop correctly. However, the molecular mechanisms behind the nuclei movement have not yet been fully resolved.
The lab of Caren Norden at the MPI-CBG together with the lab of Carl Modes at the Center for Systems Biology Dresden (CSBD), studied PSE of the developing zebrafish to get a mechanistic understanding of this nuclear positioning phenomenon. The scientists compared two differently shaped neuroepithelia. This comparative approach combined with high-resolution light sheet imaging allowed for the discovery of the molecular mechanisms and a role of tissue architecture in this cellular process. The team found that depending on tissue architecture, different actin – a key cytoskeletal protein – dependent mechanisms move nuclei to apical positions. Caren Norden’s team discovered a novel pushing mechanism that moves nuclei within the hemispheric neuroepithelium of the zebrafish retina. Carl Modes from the neighboring CSBD contributed a theoretical model that showed that such pushing mechanisms could generate enough force to move nuclei. However, in cuboidal hindbrain neuroepithelia a contractile mechanism is at play showing that tissue architecture can influence the choice of intracellular mechanisms.
Deciphering cell biology in vivo is an important aspect to understand how cells form tissues. The next steps will be to understand how tissue arrangements influence intracellular processes in other tissues and organisms. To this end, a combination of in vivo approaches, 3D culture and possibly organoids can lead a bright way into the future to cross the boundaries between cell and developmental biology. Moreover, to fully understand a phenomenon, it is crucial to also embrace interdisciplinary approaches and underline experimental finding by theory.
Iskra Yanakieva, Anna Erzberger, Marija Matejčić, Carl D. Modes, Caren Norden: Cell and tissue morphology determine actin-dependent nuclear migration mechanisms in neuroepithelia.
The Journal of Cell Biology Aug 2019, jcb.201901077; DOI: 10.1083/jcb.201901077