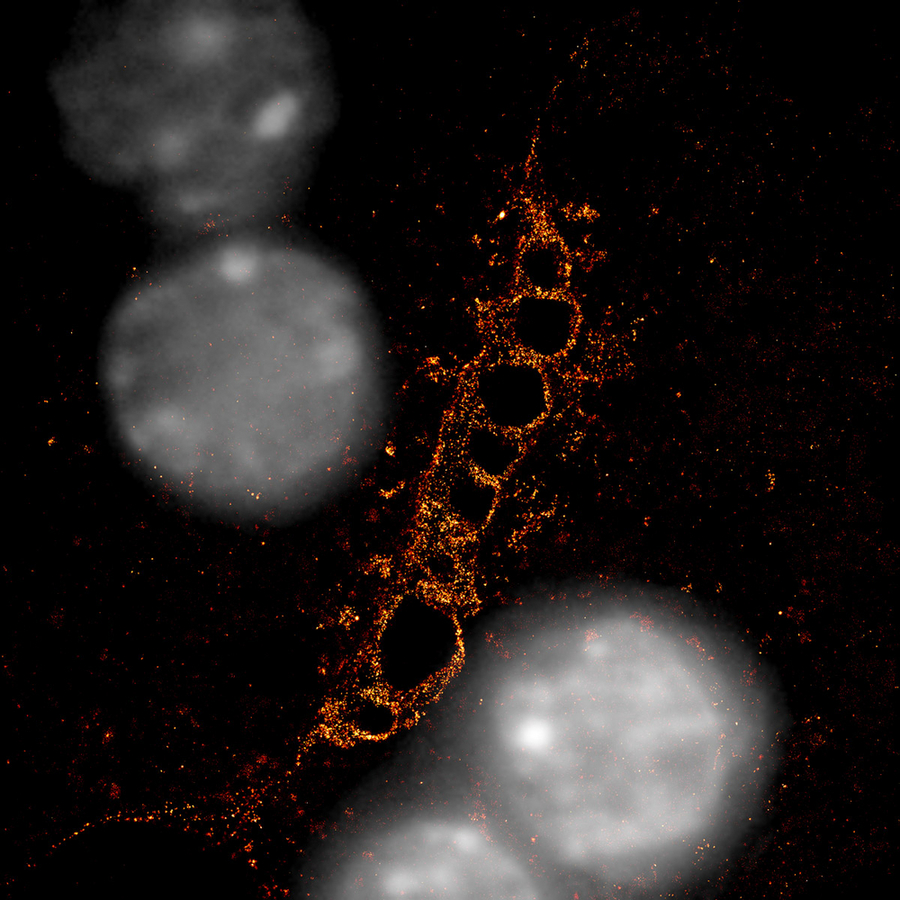
Super resolution microscopy image shows a bile canaliculus (in red), formed by two hepatocytes with their nuclei in grey. Single-molecule localization microscopy was used to analyze F-actin (red). F-actin-rich structures create a striped pattern. Copyright: Belicova et al. / MPI-CBG
The liver is our largest metabolic organ vital for detoxification and digestion. The liver produces bile, a digestive fluid that is drained into the intestine. To transport bile, liver cells form a network of tiny tubules (bile canaliculi) essential for organ function. Researchers at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden have discovered that liver cells use membranous transversal connections to reinforce the bile tubules between cells, a principle similar to bulkheads employed in boat manufacturing. These tiny membrane structures seem to be important in the development of the bile network and therefore liver function. Blockage of this network leads to a wide range of severe liver diseases. In the future, the team of Marino Zerial at the MPI-CBG will explore the possibilities to use these microscopic membrane bulkheads as a potential novel diagnostic marker for liver disease.
Our central metabolic organ is the liver. In order to digest fats and excrete waste products, liver cells - called hepatocytes - produce bile that is drained through a tubular network to bile ducts that finally disembogue into the intestine. Hepatocytes make up about 80 percent of liver mass and form a very complex three-dimensional tubular system to secrete and transport the bile. The finest ramifications of this tubular network, the bile canaliculi, are formed in between two neighboring hepatocytes and are only about one micrometer in diameter. As a comparison, human hair is about 100 micrometers wide. Because hepatocytes are organized in a three-dimensional tissue, they interact with multiple neighboring cells in all directions and form a complex network of highly ramified bile canaliculi.
Since this network is essential for liver function and human health, there have been substantial efforts from researchers to understand how hepatocytes shape their membrane surface to create such a fine and extensive network. To uncover the mechanisms that control the shape of bile canaliculi, scientists around the research group leader and director Marino Zerial at the MPI-CBG analyzed the microarchitecture of the forming network in detail.
Same principles as the bulkheads of boats
The MPI-CBG in Dresden disposes of state-of-the-art microscopy technologies that are able to visualize cell features that were previously undetectable due to their small size or fast movements. In this study, Lenka Belicova, the leading author of the study and former PhD student in the research group of Marino Zerial, explains: “We followed the embryonic hepatocytes under the microscope. They began forming an initial cavity between two adjacent cells, which was initially spherical but then grew into an elongating tube. Other cell types would typically form spherical cavities in vitro in a test tube outside of their biological context, so we wondered why hepatocytes could achieve such a tubular growth.” The observations from the movies were combined with a well-known method to take static but even more detailed images, called electron microscopy. The group leader Marino Zerial explains: “The key was the interpretation of these electron microscopy images. We discovered that neighboring hepatocytes assemble a pattern of specific extensions of their outer membrane that connected the two adjacent cells across the elongating space. These previously unrecognized structures form a pattern that reminded us of the bulkheads of boats, ships and planes. In boats, the bulkheads provide structural stability and rigidity. In the hepatocytes, they ensure the elongation of the tubes.”
In collaboration with Timofei Zatsepin, Associate Professor at the Skolkovo Institute of Science and Technology in Moscow, the researchers looked for molecular components required for the formation of the elongated tubes. Lenka adds: “We found that the gene Rab35 plays a role in shaping the elongated tubes. When Rab35 was missing, the bulkheads disappeared and the cavities formed by the hepatocytes converted into a spherical shape.” The researchers were able to demonstrate the physiological importance of the apical bulkheads. By decreasing the levels of Rab35, they could change the architecture of the developing mouse liver, causing the formation of cysts and tubes that resemble the pathological alterations observed in human diseased liver.
Implications for liver diseases
The group of Marino Zerial is the first one to describe the membrane bulkheads. The researchers were able to discover those structures with an interdisciplinary approach combining advanced microscopy techniques with computer-aided 3D reconstruction of microscopy images. This special combination made it possible to visualize the very fine details of bile canaliculi microarchitecture and make this discovery. “With the discovery of these new sub-cellular structures, we provide new insights into the longstanding problem of how the tubular bile canalicular network is formed in the embryonic liver”, summarizes Marino Zerial, who oversaw the study. “We are very excited by these findings because the apical bulkheads may hold the key for understanding liver dysfunction like cholestasis. The structural alterations of liver tissue in human patients are remarkably similar to those caused by loss of the bulkheads. Now we know where to look to interpret the progression of liver diseases, a prerequisite to develop new therapeutic strategies.”
Lenka Belicova, Urska Repnik, Julien Delpierre, Elzbieta Gralinska, Sarah Seifert, José Ignacio Valenzuela, Hernán Andrés Morales-Navarrete, Christian Franke, Helin Räägel, Evgeniya Shcherbinina, Tatiana Prikazchikova, Victor Koteliansky, Martin Vingron, Yannis Kalaidzidis, Timofei Zatsepin and Marino Zerial: “Anisotropic expansion of hepatocyte lumina enforced by apical bulkheads”, J Cell Biol (2021) 220 (10): e202103003, doi: 10.1083/jcb.202103003
Media Contact MPI-CBG
Katrin Boes
+49 (0) 351 210 2080
kboes(at)mpi-cbg.de
Scientific contact:
Prof. Marino Zerial
+49 (0) 351 210 1100
zerial(at)mpi-cbg.de