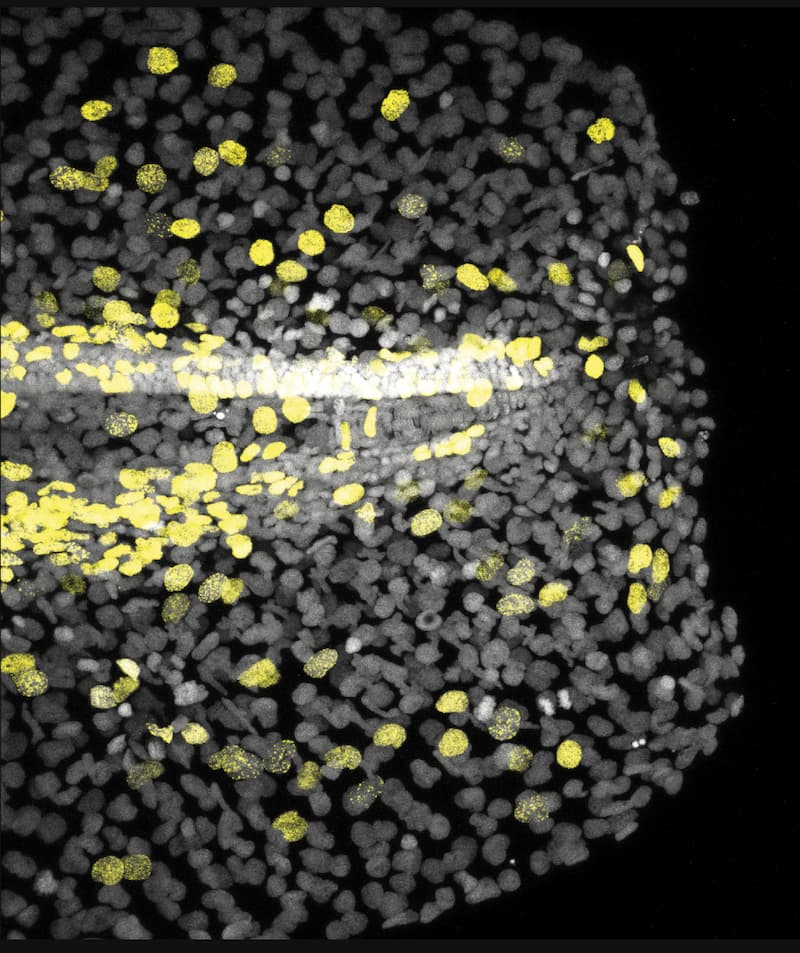
Zebrafish larval fin at 2 days-post-fertilization, 1 hour after cutting, with immunostainings labeling the nucleus of proliferative cells (yellow, EdU+) and all cells (grey, DAPI+). © Elisa Nerli
A growing field of interest in both the developmental biology and biophysics research communities is bioelectricity. Historically, bioelectrical currents have been associated with cells that produce action-potentials, like neurons or cardiac muscle. However, new findings reveal that electrical signals in all cells, not just neurons, help control how organs regenerate. This insight may transform our understanding of wound healing and organ growth.
Researchers from the group of Rita Mateus at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) and the Cluster of Excellence Physics of Life (PoL) at TU Dresden, harnessed the power of zebrafish larvae, whose tail fins can regrow rapidly following injury. Their findings, now out in Science Advances show how organs heal based on changes in membrane potential, which along with intracellular signaling, activate tissue-wide cell proliferation.
Electrical and Chemical Signals in Response to Injury
“We had an interest in combining a physics framework with biology in the context of organ injury. By cutting the tail fin of a larval zebrafish, we aimed to understand what electrical signals are generated, and how do these signals facilitate repair?” said Jinghui Liu, a postdoctoral researcher in the Mateus group, and one of the lead authors of the study. Alongside Elisa Nerli, a fellow postdoctoral researcher in the Mateus group and the study’s other co–first author, they used cutting-edge live imaging coupled to laser microdissection to map the sequence of electrical and biochemical events following injury of larval fins. The larval zebrafish tail fin model offers a unique window into this phenomenon: its thin structure and optical transparency allow researchers to precisely injure the full organ, while quantifying cell behavior at the single cell scale. Surprisingly, already at 100 milliseconds after injuring the fin, the researchers detected a collective change of the cells’ membrane potential: cells across 200 microns of the fin became ‘more positive’, their membrane potential was depolarized. This electrical signal was followed by a tissue-wide intracellular calcium wave that propagated over a few seconds. Interestingly, the calcium wave’s progression slowed down as it moved away from the injury, following the same progression dynamics as expected for the ion flux exchange between inside of the fish and freshwater medium outside.
Understanding the Origin of Electrical Signals in Tissues
The team combined experiments with physical theory by developing a new electro-diffusive model, in tight collaboration with Charlie Duclut (Sorbonne Université) and Amit Vishen in the group of co-corresponding author Frank Jülicher at the Max Planck Institute for the Physics of Complex Systems (MPI-PKS), revealing how ion flows and electric potentials interact across the tissue’s interstitial space. The epithelium acts like a leaky capacitor, allowing ions to move and creating complex electrical environments that regulate signaling. By manipulating the ionic environment, immersing fish in high potassium solutions or using genetic mutants with altered ion channel conductance, the researchers could remodel the fin wound currents and change its regenerative response. These experiments confirmed that bioelectric signals are not only necessary but can be modulated to influence organ healing and growth.
Electrical signals, chemical signals, and their interpreter: Voltage Sensing Phosphatase
But how do cells relay the electrical signal to trigger proliferation? A key discovery was the identification of the transmembrane protein Voltage Sensing Phosphatase (VSP) as the sensor that detects the injury-induced changes in membrane potential. When activated by the electrical signal at the cell membrane, VSP changes conformation and triggers intracellular pathways that promote widespread cell proliferation, fueling tissue regrowth. “We wondered whether this protein was necessary and/or sufficient to trigger cell proliferation in response to membrane depolarization. Being widespread across the animal kingdom, it could point to a common growth and repair mechanism among different species” said Elisa Nerli, co-first author of the study.
Using CRISPR technology, the team generated zebrafish mutants lacking VSP, which fail to relay the electric signal inside cells, resulting in impaired fin regeneration. Remarkably, overexpressing VSP was sufficient to lead to larger organs, highlighting its pivotal role in translating electrical cues into organ growth. “Altogether, we found that the rapid onset of proliferation upon damage is a direct consequence of spatiotemporal electrochemical coupling. This opens exciting new frontiers into thinking how membrane potential mechanistically contributes to regulating organ size and scaling”, said Rita Mateus, the co-corresponding author of the study and joint group leader at MPI-CBG and PoL.
“Altogether, we found that the rapid onset of proliferation upon damage is a direct consequence of spatiotemporal electrochemical coupling. This opens exciting new frontiers into thinking how membrane potential mechanistically contributes to regulating organ size and scaling”
Rita Mateus
Implications for Regenerative Medicine
Bridging physics and biology, this collaborative study reveals how global electrical signals and specific chemical molecules are intertwined to coordinate organ repair. The findings provide a new general framework for understanding wound healing and organ growth – suggesting that bioelectric modulation could become a powerful tool in tissue engineering. By characterizing the hidden electrical language of injured organs, this study opens avenues to rationally employ new therapies harnessing bioelectric signals to enhance tissue and organ healing.
News article in Chinese language from BioArt
News Article in Chinese Language: Download PDF
Jinghui Liu#, Elisa Nerli#, Charlie Duclut, Amit S. Vishen, Naomi Berbee, Sylvia Kaufmann, Cesar Ponce, Aristides B. Arrenberg, Frank Jülicher*, Rita Mateus*. Injury-induced electrochemical coupling triggers organ growth. Science Advances (2026). DOI: https://doi.org/10.1126/sciadv.aec0687