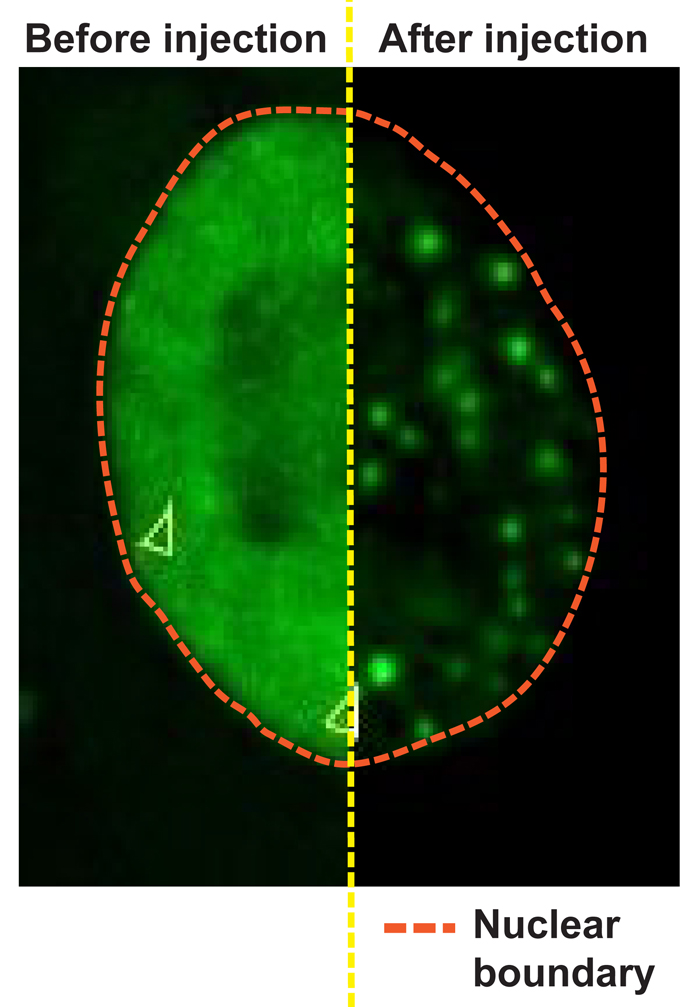
Monatge of half nucleus of untreated HeLa cell expressing FUS-GFP, and the other half of the same cell after microinjection of RNAse A.
Many age-related diseases affect the nervous system. One prominent example of a neurodegenerative disease is Amyotrophic Lateral Sclerosis (ALS). The brain tissue of ALS patients typically shows aggregates of so-called prion-like RNA-binding proteins. In the nucleus, these RNA-binding proteins are floating in solution, but when they are located outside of the nucleus, in the cytoplasm, they often form solid pathological aggregates. Researchers from the lab of Simon Alberti and Tony Hyman at the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden wanted to investigate why these proteins never aggregate in the nucleus and what keeps them soluble there? Uncovering this mechanism may help to dissolve pathological aggregates in the cytoplasm.
The researchers had a closer look at the nucleus and found a high concentration of RNA. Could this high concentration of RNAs keep disease-associated RNA-binding proteins in solution? Shovamayee Maharana, the first author of the study, explains: “Prion-like RNA-binding proteins are binding to RNA to perform a certain function. To perform that function, they often form transient assemblies which quickly dissolve when the job is done. In pathological states these assemblies do not dissolve anymore and convert into rigid aggregates. But how does RNA binding change this transition?” In order to test the hypothesis whether lots of RNA in the nucleus keeps these proteins in a soluble state, the researchers injected enzymes in the nucleus, which chops RNA into pieces so that nothing can bind. As a result, the RNA-binding proteins were set free and formed aggregates! “We next asked ourselves if the presence of RNA in assemblies will keep them in dynamic physiological state and prevent the transition to rigid aggregates and to our surprise, it worked both in cells and test tubes.”, tells Shovamayee.
Simon Alberti, research group leader, has a theory: “When we age, the balance of RNA and prion-like proteins could be disturbed and we think that that as we age, the nuclear RNA concentration decreases. This could trigger the formation of pathological aggregates”. The researchers still don’t understand what happens in neurons when they age. This needs further studies to generate new therapies for diseases like ALS. This study represents an important step towards that goal.
The study will be of particular interest to those researchers working on age-related neurodegenerative disease. More generally, the idea that RNA acts as a buffer, provides exciting links between RNA biology and the physical chemistry of proteins.
Shovamayee Maharana, Jie Wang, Dimitrios K. Papadopoulos, Doris Richter, Andrey Pozniakovsky, Ina Poser, Marc Bickle, Sandra Rizk, Jordina Guillén-Boixet, Titus Franzmann, Marcus Jahnel, Lara Marrone, Young-Tae Chang, Jared Sterneckert, Pavel Tomancak, Anthony A. Hyman, Simon Alberti: RNA buffers the phase separation behavior of prion-like RNA binding proteins, Science, 12 Apr 2018